Introduction
The ongoing novel coronavirus disease 2019 (COVID-19) pandemic has provoked questions about treatment decisions in patients with hematologic malignancies. Of note, several anti-cancer agents have been shown to have preclinical activity against the SARS-CoV-2 virus, including ruxolitinib (Jakafi), as well as the hypomethylating agents (HMA) decitabine (Dacogen) and azacitidine (Vidaza). In addition, ruxolitinib has previously been shown to have potent activity against the cytokine response, similar to that seen in COVID-19 infections. The optimal management of patients on these agents who become infected with COVID-19 is unknown and it is not clear if the use of these agents modifies the clinical course of infection. We describe a case series of patients with hematologic malignancies receiving therapy with these agents while infected with COVID-19.
Methods
We conducted a retrospective chart review of consecutive patients between March 1 and May 21, 2020 who were receiving therapy with ruxolitinib, azacitidine, or decitabine when diagnosed with COVID-19 by SARS-CoV-2 PCR assay within the Mount Sinai Health System. The indications for these agents included acute lymphocytic leukemia, acute myeloid leukemia, myelofibrosis, polycythemia vera, and myelodysplastic syndromes. Patients who last received the agent more than thirty days prior to COVID-19 diagnosis were excluded from our series.
Results
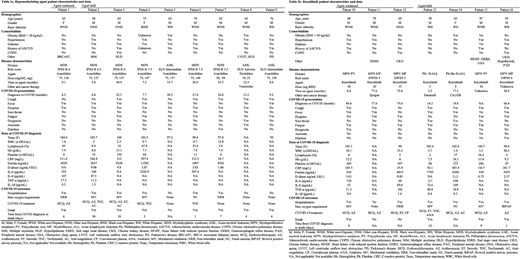
Sixteen patients on HMA or ruxolitinib were diagnosed with COVID-19 within our health system during this span. Full patient characteristics and clinical data are described in Tables 1a and 1b. Seven patients were receiving ruxolitinib and nine patients were on HMA: eight on azacitidine and one on decitabine. Of the patients on HMA, eight had therapy held after confirmed COVID-19 infection, and one was continued on therapy. Overall mortality in the HMA cohort was 88.9% (8/9), including 7/8 in the therapy-held group and the one patient for whom therapy was continued. Of the patients on ruxolitinib, three had therapy held and four were continued after confirmed infection. Overall mortality in the ruxolitinib cohort was 57.1% (4/7), with 75% (3/4) in the therapy-held and 33% (1/3) mortality in the therapy-continued groups. All patients who died (n = 12) required either non-invasive positive pressure ventilation or mechanical ventilation. Across all agents, patients who survived (n = 4) required no higher level of supplemental oxygen than non-rebreather mask, and one did not require hospitalization. Cytokine levels were on average higher on admission for the ruxolitinib-held group but were not associated with mortality or increased oxygen requirement.
Discussion
High mortality rates were observed for both ruxolitinib and HMA in this high-risk group of patients with hematologic malignancy regardless of whether therapy was held. HMA was commonly held at time of infection: further investigation will be needed to elucidate any potential in-vivo anti-SARS-CoV-2 activity for these reagents. Mortality was numerically less in patients in receiving ruxolitinib who continued therapy, however this should be interpreted with caution given our small cohort. Further clinical trials to address the use of ruxolitinib are necessary and are ongoing. These data can help guide further investigation regarding use of anti-cancer therapy in patients with hematologic malignancy diagnosed with COVID-19.
Mascarenhas:Celgene, Prelude, Galecto, Promedior, Geron, Constellation, and Incyte: Consultancy; Incyte, Kartos, Roche, Promedior, Merck, Merus, Arog, CTI Biopharma, Janssen, and PharmaEssentia: Other: Research funding (institution).
Author notes
Asterisk with author names denotes non-ASH members.